Abstract
Introduction: Somatic gene mutations involved in DNA methylation, chromatin modification, RNA splicing, DNA transcription/signal transduction contribute to the development of myelodysplastic syndrome (MDS). Risk stratification and therapeutic decision-making of MDS are based on the International Prognostic Scoring System-Revised (IPSS-R), which considers hematologic parameters and cytogenetic abnormalities. Recently, Bernard et al developed IPSS-Molecular (IPSS-M) in order to improve risk stratification of patients with MDS by combining genomic profiling.
We aim to evaluate MDS mutation profiling and investigate the IPSS-M in daily clinical practice
Methods: Single-center retrospective study included a total of 41 patients diagnosed with MDS and were profiled with a Custom Myeloid Panel (ThermoFisher in peridiagnostic samples, between January 2016 to May 2022. Clinical characteristics, including hematologic parameters, risk stratification, morphologic and cytogenetic abnormalities were collected. We then assessed prognosis (Leukemia free survival, LFS, and overall survival, OS) of IPSS-M risk groups, comparing with previous IPSS-R.
Results: A total of 41 patients were analyzed, with a median age 67(25-86) years, 18 were (43.9%) males. Twenty-six (63.4%) patients presented Hb<10g/dL, 22 (53.7%) Platelets <100 x109/L and 7 (17.1%) absolute neutrophil count <0.8 x109/L and 16 (39.0%) had 2 or more cytopenias.Cytogenetics classification was good in 27 (65.9%) patients, intermediate in 8 (19.5%) and very poor in 3 (7.3%). Medullary blasts were ≤2% in 18 (43.9%) patients, >2-<5 in 5 (12.2%), ≥5-≤10 in 8 (19.5%) and >10 in 10 (24.4%).
Eighy-five percent of patients (n=35, 85.4%) had at least one mutation and 31.7% (13) patients 3or more mutations and presented a worse prognosis evaluated by LFS (HR 3.6(1.1-12.7), P=0.035) and OS (HR 3.2(1.4-7.7), P=0.035).
The most common mutations were SF3B1, TET2, ASXL1 and JAK2, accounting for 13.3%, 12.0%, 12.0%, 12.0% and 8.4%, respectively. Mutations in TP53 (HR 4.4[1.4-13.9], P=0.01), ASXL1 (HR 3.0[1.1-8.3], P=0.035), U2AF1 (HR 22.5[3.6-140.5], P= 0.001), NRAS/KRAS (HR 22.7[4.5-115.9], P= 0.001) were significantly associated with adverse outcome in OS, but only ASXL1 were statistically predictor of LFS (HR 5.0[1.2-21.0], P=0.026).
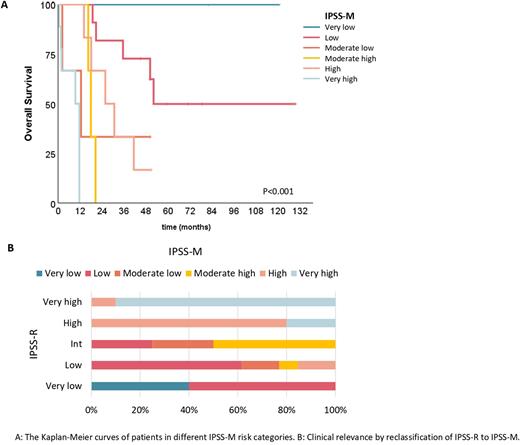
According to the IPSS-R categories, most of patients were low risk (n=13, 35.1%) or very high risk (n=10, 27.0%). Regarding the mutation risk stratification (IPSS-M), 12 (32.4%) patients were low risk, 7 (18.9%) high risk and 10 (27.0%) very high risk. The OS was significantly different between IPSS-R and IPSS-M. The median OS per IPSS-R score was NA, 41.4, 16.4, 25.8, 11.6 months for very low-, low-, intermediate-, high-, and very high-risk, respectively, P<0.001. In IPSS-M score, the median OS was NA, 52.1, 12.6, 17.9, 25.8 and 9.4 months for very low-, low-, mod low, mod high, high-,and very high-risk, respectively, P<0.001. The prediction ability of prognosis by IPSS-R and IPSS-M was assessed by the area under the curve (AUC)of ROC with OS in R. The AUC value of the IPSS-M was higher than IPSS-R (0.630vs0.564).
We evaluate the clinical significance of the IPSS-M, comparing the risk stratifications changes and after merging moderate low and moderate high into moderate: 11 (29.7%) patients were reclassified, 10 (27.0) were upstage and 1 (2.7%) downstage. After reclassification according to IPSS-M, 5 (13.5%) patients would benefit from treatment strategies.
Conclusions: In our cohort, 85% of MDS patients had at least one mutation and the number of abnormalities correlated with disease severity, as well mutations in TP53, ASXL1, U2AF1 and NRAS/KRAS. IPSS-M, the novel system integrating hematologic, cytogenetic parameters and gene mutations,improved the risk stratification of patients with MDS, representing a valuable toolfor best treatment in patient-centered approach.
Disclosures
Geraldes:Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene/BMS: Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.